HBcAb Rapid Test Strip
HBcAb Rapid Test Strip (乙型肝炎核心抗体(HBcAb)血清检测试纸条) is a one-step qualitative lateral flow rapid diagnostic test kit, based on the Colloidal Gold Chromatographic Immuno Competitive Binding principle, which is used to detect Anti Hepatitis-B Core antigen in human Serum specimen.
Supplementary information of HBcAb Rapid Test Strip
- General and Technical information of this HBc test kit;
- Packing specification of this HBc Dipstick style test kit (gross weight in full kit package and in bulk packing);
- Specimen used to carry out the assay, and the requirements on specimen collection, storage and processing; and,
- Specific immuno assay principle used in this Hepatitis B Test Kit.
Main technical information of this HBcAb test kit is listed in the following table:
| Former Cat. No.: | HBV 251 |
|---|---|
| Description: | HBcAb Rapid Test Strip |
| Category: | Hepatitis Disease |
| Style: | Dipstick |
| Unit: | PCS |
| Specimen: | Serum |
| No. of Step: | One Step |
| Reading Time: | 10-20 Minutes |
| Cut Off: | 2 ng/ml |
| Specification: | 3.0 mm |
| Quan. or Qual.: | Qualitative |
| Sensitivity: | 96.1% |
| Specificity: | 98.2% |
| Assay Principle: | Colloidal Gold Chromatographic Immuno Competitive Binding |
The standard kit size of this Hepatitis B Dipstick test kit is 100 tests per box; 48 boxes per carton.
To save freight cost, sometimes bulk packing is used, meaning test strip, cassette or multi-test panel is packed in single aluminum pouch; 50 or 100 pouches in one plastic bag; and then plastic bags are packed in cartons along with the unfolded boxes for the test kits. In this way, the customers can easily pack the tests into test kits after receiving the bulk packed goods.
| Tests per Kit: | 100 Tests |
|---|---|
| Dimension of Kit Box: | 21.0 * 12.5 * 5.5 CM |
| Boxes per Carton: | 48 Kits |
| Tests per Carton (BULK): | 6000 Tests |
| G.W. per Carton (Boxed): | 15.0 KG |
| G.W. per Carton (Bulk): | 16.0 KG |
| Carton Dimension: | 53 * 45 * 36 CM |
| Carton Volume: | 0.085 CBM |
Materials Provided
- Lateral Flow immunochromatographic assay (GICA) Strip;
- Instruction leaflet;
- Disposable urine cup (optional).
To carry out the assay with a HBcAb Rapid Test Strip, Serum should be collected according to the instructions given in the Instruction For Use included in the test kit. After collecting, it is preferable to perform the testing as early as possible; if not viable, the specimen should be stored properly. A general introduction about the collection, and storage of the specimens is given below.
Serum Collection
Collect blood specimen into a red top blood collection tube by vein puncture, which contains no anticoagulants.
Allow the blood to clot, or separate the serum by centrifugation.
Carefully withdraw the serum into a new pre-labeled tube.
Serum Storage Condition
Test specimens as soon as possible after collecting. The specimens can be stored up to 5 days at 2-8°C if not tested immediately. The specimens should be frozen at -20°C for longer time storage. Don’t freeze and thaw the specimens for many times. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible particulate matter should be clarified by centrifugation before testing. Do not use samples demonstrating gross lipemia, gross hemolysis or turbidity in order to avoid interference on result interpretation.
Colloidal Gold Chromatographic Immuno Competitive Binding method is used in this HBcAb Rapid Test Strip to detect the Anti Hepatitis-B Core antigen in human Serum specifically. The introduction of this Colloidal Gold Chromatographic Immuno Competitive Binding testing principle is given blow.
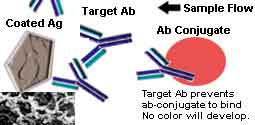
Drug abuse test kits are based on the Lateral Flow Competitive Binding rapid test principle.
In the test kit, drug-protein conjugates (purified bovine albumin) are immobilized in the test line region. And the drug specific antibody conjugated with colloidal gold particles is put in the membrane of the sample adding region.
During testing, when the urine specimen is added to the sample adding region, the antibody conjugate particles will come into the urine specimen, where the antibody conjugate will react with the drug. If there is no target drug in the urine specimen, or the concentration is lower than the cut-off, the drug can not bind all the binding sites of the drug specific antibody colloidal gold conjugate.
The unbonded colloidal gold conjugate will be captured by the immobilized drug in the testing line region. When the antibody conjugated colloidal gold particle congregate, a red or pink color will develop, indicating a negative result.
If the concentration of the drug is higher than the cut off value of the test kit, the drug will saturate all the binding sites of the antibody conjugate. Then, when the mixed fluid moves to the test region under capillary effect, there will be no free antibody particles to bind with the immobilized drug, and no color will develop, indicating a positive result.

 Atlas Link Technology Co., Ltd
Atlas Link Technology Co., Ltd